| ||||
Diffusion, Osmosis & Tonicity - P2
This is also why, if stranded at sea, people are cautioned not to drink the ocean water, no matter how thirsty they may become.
SPO VIRTUAL CLASSROOMS
PAGE 2 < Back to Page 1
Continued >
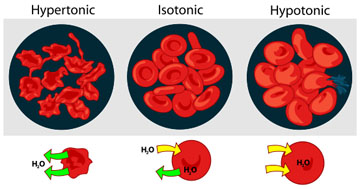
Osmosis and Animal Cells
The cells of our body normally exist in an isotonic environment. When cells are placed in a hypertonic environment (higher concentration of solutes than the cell), water leaves the cell and the cell becomes shriveled.
Conversely, when animal cells are placed in a hypotonic solution (lower concentration of solutes than the cell), water moves into the cell, and unlike plant or bacteria cells that have a sturdy cell wall, the animal cell swells and may explode. This is why a nurse can't give someone an IV drip of pure water. The patient's cells would pop!
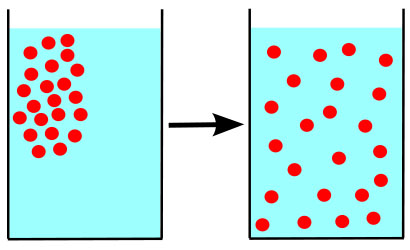
What Is Osmosis?
Osmosis is a specific kind of diffusion; the diffusion of water molecules across a membrane, typically the membrane of a living cell.
The environment surrounding each of our cells may contain small amounts of dissolved substances (solutes) that are equal to, less than, or greater than those found within the cell.
The relationship between the concentrations of solutes on either side of the membrane is referred to as tonicity.
Page last updated: 3/2016
How Does Tonicity Relate to Osmosis?
If a cell is in a surrounding environment that's:
- isotonic: There is no net movement of water between cell and environment. The concentration of solutes is the same on either side of the membrane.
- hypertonic: This term refers to the side of the membrane with a higher concentration of solute.
- hypotonic: This term refers to the side of the membrane with a lower concentration of solute.
These terms describing tonicity are dependent on the relationship between the environments on either side of the membrane, and can apply to the environment inside the cell or the environment outside the cell. The key to understanding osmosis and tonicity is to remember that water will always move toward a hypertonic environment!
Drinking salt water actually robs the body of hydration, because it creates a hypertonic environment in the GI tract, which pulls water out of our cells, dehydrating the body.
Membrane Transport Video
"In Da Club"
from
Crash Course Biology - FUN!
 | ||||
Science Prof Online
has several
Virtual Classrooms
including:
(15 week)
(15 week)
(8 week)
(8 week)
(15 week)
 | ||||||
See all of related teaching materials on the