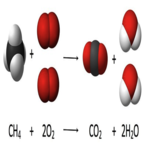
| ||||
Chemical Bonds & Reactions
Review Questions
Virtual Cell Biology Classroom of Science Prof Online
Chemical Bonds & Reactions Review Qus
 | ||||
Free review questions to help students better understand
 | ||||||
SPO VIRTUAL CLASSROOMS
The following questions, from the Virtual Cell Biology Classroom, are designed to help students better understand Inorganic Chemistry. All questions are based on materials that can be found on the Chemical Bonds & Reactions Lecture Main Page.
1. Name and describe the 3 types of chemical bonds that we discussed in class.
2. What does it mean when a molecule is described as being 'polar'?
3. Explain the statement "All compounds are molecules , but all molecules aren't compounds." Give an example.
4. What types of elements tend to form covalent bonds? What type form ionic bonds?
5. Differentiate between oxidation and reduction. What event makes an atom or molecule reduced? oxidized?
6. What is the difference between a mixture and a compound?
7. A dehydration reaction is a synthesis reaction and a hydrolysis reaction is a decomposition reaction. Describe specifically how dehydration makes bigger molecules. How does hydrolysis make smaller molecules?
Page last updated:
5/2016
8. Determine whether the following chemical reaction is balanced or not. Show your work using the technique discussed in class.
SiCl4 + 4H2O ----> Si(OH)4 + 4HCl
9. The following represents three calcium carbonate molecules. How many atoms of each element are present in total?
3CaCO3
10. What are the three basic reaction types? Describe each and list all the names we learned for each reaction type (synonyms).
You have free access to a large collection of materials used in a college-level introductory Cell Biology Course. The Virtual Cell Biology Classroom provides a wide range of free educational resources including Power Point Lectures, Study Guides, Review Questions and Practice Test Questions.
You have FREE access to a large collection of materials used in a college-level introductory biology course. The Virtual Biology Classroom provides a wide range of free educational resources including PowerPoint Lectures, Study Guides, Review Questions & Practice Test Questions.